Advanced Placement Chemistry
1965 Free Response Questions
1) An acidified solution of a sample of pure oxalic acid dihydrate, H2C2O4
. 2 H2O, was used to standardize a solution of potassium
permanganate. In this process, the oxalic acid was oxidized to carbon dioxide and the
permanganate ion was reduced to manganese(II) ion. Use the following data to calculate the
molarity (formality) of the permanganate solution:
| Weight of flask and acid crystals |
68.6153 grams |
| Weight of flask |
68.4262 grams |
| Burette reading after adding KMnO4 solution |
31.52 mL |
| Burette reading before adding KMnO4 solution |
1.51 mL |
2) Two hydrocarbons, one a gas and the other a solid at room temperature, have the same
composition.
a) When either hydrocarbon is burned, the weight of carbon dioxide produced is 3.26
times the weight of water formed. Derive the empirical formula for the hydrocarbons
b) When 2.60 grams of the gas is placed in an evacuated 2.00-liter container, at 527 °C
the pressure is 1.20 x 103 millimeters of mercury. Determine the molecular
weight of the gaseous hydrocarbon.
c) A solution of 3.25 grams of the solid hydrocarbon in 40.0 grams of benzene begins to
freeze at 2.969 °C. The freezing point of pure benzene is 5.533 °C and the molal
freezing point constant for benzene is 5.128 centigrade degrees per molal. Determine the
molecular weight of the solid hydrocarbon.
3)
| Zn(s) ---> Zn2+ + 2e¯ |
E°= 0.76 volt |
| Cu(s) ---> Cu2+ + 2e¯ |
E°= -0.34 volt |
| 2.3 (RT/F) = 0.059 at 25 °C |
A cell is set up to plate zinc onto a zinc electrode. The loss of weight of a copper
electrode is used to determine the amount of current passed.
a) Assuming box A to be a source of direct current, if 100 milliliters of a solution of
Zn2+, initially at a 1.00-molar concentration, is electrolyzed until 1.00 per
cent of the original Zn2+ remains in solution,
1. how many coulombs are required?
2. how much weight does the copper electrode lose?
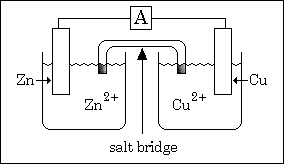 b) Assuming box A to be a voltmeter,
what is the standard potential of the cell?
b) Assuming box A to be a voltmeter,
what is the standard potential of the cell?
c) What is the minimum voltage to be applied to this cell if the electrolysis is to
continue until the concentration of Zn2+ reaches 1.00 per cent of the original?
(Assume that the concentration of Cu2+ reaches 1.00-molar and that the
temperature is 25 °C.)
Questions 4 - 14 are not available.
15) Criticize the statement. Chemical reactions tend to proceed in the direction of the
evolution of heat.
16) Explain why the spectra of gaseous atoms consist of sharp lines.
17) Give an explanation for each of the following observations:
a) The boiling point of CBr4 is distinctly higher than that of CCl4.
b) The boiling point of CH3OH is distinctly higher than that of CH3Cl.
18) In each of the following cases, a slight excess of dilute base is
added to a 0.05-molar solution of acid. The resultant heats of neutralization per mole of
acid reacting are indicated for each acid-base reaction.
| Heat of Neutralization |
| a) HCl + NaOH |
13.7 kcal. |
| b) HNO3 + KOH |
13.7 kcal. |
| c) HCl + NH3 |
12.6 kcal. |
| d) CH3COOH + NaOH |
13.4 kcal. |
| e) HClO4 + KOH |
13.7 kcal. |
Account for the fact that the heats are the same in (a), (b), and (e) but are different
in (c) and (d).
19) Give an explanation for each of the following observations:
a) Many complex ions of zinc, such as Zn(CN)42-, exhibit a
coordination number of four.
b) Complex ions of Fe(III), such as Fe(CN)63-, are paramagnetic.
20) The terms "activation energy" and "activated complex" (or
"transition state") are commonly employed in a theoretical discussions of rates
of reaction. What is the significance of these terms?
21) You are given a sample of ordinary sodium chloride, some glassware, a crucible, a
3-volt battery, an ammeter, wires, distilled water, carbon tetrachloride, a bunsen burner,
a balance, equipment for supporting a crucible or beaker, and a selection of thermometers.
Your problem is to obtain experimental evidence for the assertion that sodium chloride is
electrovalently bonded. Describe a series of relevant observations and experiments.
Explain how the concept of ionic bonding gives a consistent interpretation of all of the
observations and experiments.
22) Discuss a chemical and a physical method for the determination of atomic weights.
State what experimental data are needed and show how the data may be employed to achieve
the desired objective. Indicate any assumptions that are made in the use of each method.
b) Assuming box A to be a voltmeter,
what is the standard potential of the cell?