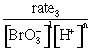* *
* Part A *
* *
Time – 40 minutes
YOU MAY USE YOUR CAL-CULATOR FOR PART A.
CLEARLY SHOW THE METHOD USED AND STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your advantage to do this, because you may earn partial credit if you do and you will receive little or no credit if you do not. Attention should be paid to significant figures. Be sure to write all your answers to the questions on the lined pages following each question in this booklet.
Answer Question I below. The Section II score weighting for this question is 20 percent.
2003 A Required
1. C6H5NH2(aq) + H2O(l) √ C6H5NH3+(aq) +
Aniline, a weak base, reacts with water according to the reaction represented above.
(a) Write the equilibrium constant expression, Kb, for the reaction represented above.
(b) A sample of aniline is dissolved in water to produce 25.0 mL of 0.10 M solution. The pH of the solution is 8.82. Calculate the equilibrium constant, Kb, for this reaction.
(c) The solution prepared in part (b) is titrated with 0.10 M HCl. Calculate the pH of the solution when 5.0 mL of the acid has been titrated.
(d) Calculate the pH at the equivalence point of the titration in part (c).
(e) The pKa values for several indicators are given below. Which of the indicators listed is most suitable for this titration? Justify your answer.
|
Indicator |
pKa |
|
Erythrosine |
3 |
|
Litmus |
7 |
|
Thymolphthalein |
10 |
Answer:
(a) Kb = 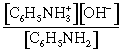
(b) pOH = 14 – pH = 14 – 8.82 = 5.18
-log[
[
Kb = ![]() = 4.4´10–10
= 4.4´10–10
(c) 25 mL ´ = 2.5 mmol C6H5NH2
5 mL ´ = 0.5 mmol H+ added
2.0 mmol base remains in 30.0 mL solution
4.4´10–10 = 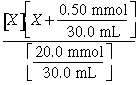
X = 1.80´10–9 = [
[H+]
= ![]() = 5.6´10–6; pH = 5.26
= 5.6´10–6; pH = 5.26
(d) when neutralized, there are 2.5 mmol of C6H5NH3+ in 50.0 mL of solution, giving a [C6H5NH3+] = 0.050 M
this cation will partially ionize according to the following equilibrium:
C6H5NH3+(aq) √ C6H5NH2(aq) + H+(aq)
at equilibrium, [C6H5NH2] = [H+] = X
[C6H5NH3+] = (0.050–X)
![]() = Ka
= 2.3´10-5
= Ka
= 2.3´10-5
X = 1.06´10–3 = [H+]
pH = –log[H+] = 2.98
(e) erythrosine; the indicator will change color when the pH is near its pKa, since the equivalence point is near pH 3, the indicator must have a pKa near this value.
Answer EITHER Question 2 below OR Question 3. Only one of these two questions will be graded. If you start both questions, be sure to cross out the question you do not want graded. The Section II score weighting for the question you choose is 20 percent.
2003 B
2. A rigid 5.00 L cylinder contains 24.5 g of N2(g) and 28.0 g of O2(g)
(a) Calculate the total pressure, in atm, of the gas mixture in the cylinder at 298 K.
(b) The temperature of the gas mixture in the cylinder is decreased to 280 K. Calculate each of the following.
(i) The mole fraction of N2(g) in the cylinder.
(ii) The partial pressure, in atm, of N2(g) in the cylinder.
(c) If the cylinder develops a pinhole-sized leak and some of the
gaseous mixture escapes, would the ratio ![]() in the cylinder increase, decrease, or remain the same?
Justify your answer.
in the cylinder increase, decrease, or remain the same?
Justify your answer.
A different rigid 5.00 L cylinder contains 0.176 mol of NO(g) at 298 K. A 0.176 mol sample of O2(g) is added to the cylinder, where a reaction occurs to produce NO2(g).
(d) Write the balanced equation for the reaction.
(e) Calculate the total pressure, in atm, in the cylinder at 298 K after the reaction is complete.
Answer:
(a) 24.5 g N2 ´ = 0.875 mol N2
28.0 g O2 ´ = 0.875 mol O2
P = = ![]()
= 8.56 atm
(b) (i) ![]() = 0.500 mole fraction
N2
= 0.500 mole fraction
N2
(ii) ![]()
= 8.05 atm ´ mole fraction = 8.05 atm ´ 0.500
= 4.02 atm N2
(c) decrease; since N2 molecules are lighter than O2 they have a higher velocity and will escape more frequently (Graham’s Law), decreasing the amount of N2 relative to O2
(d) 2 NO + O2 ® 2 NO2
(e) all 0.176 mol of NO will react to produce 0.176 mol of NO2, only 1/2 of that amount of O2 will react, leaving 0.088 mol of O2, therefore, 0.176 + 0.088 = 0.264 mol of gas is in the container.
P = = ![]()
= 1.29 atm
2003 B
5 Br–(aq) + BrO3–(aq) + 6 H+(aq) ® 3 Br2(l) + 3 H2O(l)
3. In a study of the kinetics of the reaction represented above, the following data were obtained at 298 K.
|
Experiment |
Initial
[Br–]
(mol L-1) |
Initial
[BrO3–] (mol L-1) |
Initial
[H+]
(mol L-1) |
Rate of
Disappearance of BrO3– (mol L-1 s-1) |
|
1 |
0.00100 |
0.00500 |
0.100 |
2.50´10-4 |
|
2 |
0.00200 |
0.00500 |
0.100 |
5.00´10-4 |
|
3 |
0.00100 |
0.00750 |
0.100 |
3.75´10-4 |
|
4 |
0.00100 |
0.01500 |
0.200 |
3.00´10-3 |
(a) From the data given above, determine the order of the reaction for each reactant listed below. Show your reasoning.
(i) Br–
(ii) BrO3–
(iii) H+
(b) Write the rate law for the overall reaction.
(c) Determine the value of the specific rate constant for the reaction at 298 K. Include the correct units.
(d) Calculate the value of the standard cell potential, E˚, for the reaction using the information in the table below.
|
Half-reaction |
E˚ (V) |
|
Br2(l) + 2e- ® 2 Br–(aq) |
+1.065 |
|
BrO3–(aq) + 6 H+(aq) + 5e- ® 1/2 Br2(l) + 3 H2O(l) |
+1.52 |
(e) Determine the total number of electrons transferred in the overall reaction.
Answer:
(a) (i) 1st order with respect to Br–; in experiments 1 and 2, a doubling of the [Br–] results in the doubling of the initial rate, and indication of 1st order
(ii) 1st order with respect to BrO3–
using expt. 1 & 3
rate1 = k[Br–]1[BrO3–]m[H+]n
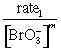 = k[Br–]1[H+]n
= k[Br–]1[H+]n
rate3 = k[Br–]1[BrO3–]m[H+]n
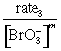 = k[Br–]1[H+]n
= k[Br–]1[H+]n
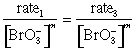
![]()
m = 1
(iii) 2nd order with respect to H+
using expt. 3 & 4
rate3 = k[Br–]1[BrO3–]1[H+]n
rate4 = k[Br–]1[BrO3–]1[H+]n
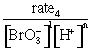 = k[Br–]1
= k[Br–]1
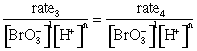
n = 2
(b) rate = k[Br–]1 [BrO3–]1 [H+]2
(c) 2.50x10–4 = k (0.00100) (0.00500) (0.100)2
k = 5000 mol–3L3s-1
(d) E˚ = 1.52 + -1.065 V = 0.455 V
(e) the overall reaction can be made by reversing the first half-reaction and multiplying by 2.5, therefore, there are 5 electrons transferred.
* * * Part B
* * *
Time — 50 minutes
NO CALCULATORS MAY BE USED FOR PART B.
Answer Question 4 below. The Section II score weighting for this question is 15 percent.
Your responses to the rest of the questions in this part of the examination will be graded on the basis of the accuracy and relevance of the information cited. Explanations should be clear and well organized. Examples and equations may be included in your responses where appropriate. Specific answers are preferable to broad, diffuse responses.
2003 C
4. Write the formulas to show the reactants and the products for any FIVE of the laboratory situations described below. Answers to more than five choices will not be graded. In all cases, a reaction occurs. Assume that solutions are aqueous unless otherwise indicated. Represent substances in solution as ions if the substances are extensively ionized. Omit formulas for any ions or molecules that are unchanged by the reaction. You need not balance the equations.
(a) A solution of potassium phosphate is mixed with a solution of calcium acetate.
PO43– + Ca2+ ® Ca3(PO4)2
(b) Solid zinc carbonate is added to 1.0 M sulfuric acid.
ZnCO3 + H+ ® Zn2+ + H2O + CO2
(c) A solution of hydrogen peroxide is exposed to strong sunlight.
H2O2 ® H2O + O2
(d) A 0.02 M hydrochloric acid solution is mixed with an equal volume of 0.01 M calcium hydroxide.
(e) Excess concentrated aqueous ammonia is added to solid silver chloride.
NH3 + AgCl ® [Ag(NH3)2]+ + Cl–
(f) Magnesium ribbon is burned in oxygen.
Mg + O2 ® MgO
(g) A bar of strontium metal is immersed in a 1.0 M copper(II) nitrate solution.
Sr + Cu2+ ® Cu + Sr2+
(h) Solid dinitrogen pentoxide is added to water.
N2O5 + H2O ® H+ + NO3–
Your responses to the rest of the question in this part of the examination will be graded on the basis of the accuracy and relevance of the information cited. Explanations should be clear and well organized. Examples and equations may be included in your responses where appropriate. Specific answers are preferable to broad, diffuse responses.
Answer BOTH Question 5 below AND Question 6. Both of these questions will be graded. The Section II score weighting for these questions is 30 percent (15 percent each).
2003 D Required
5. A student is instructed to determine the concentration of a solution of CoCl2 based on absorption of light (spectrometric/colorimetric method). The student is provided with a 0.10 M solution of CoCl2 with which to prepare standard solutions with concentrations of 0.020 M, 0.040 M, 0.060 M and 0.080 M.
(a) Describe the procedure for diluting the 0.10 M solutions to a concentration of 0.020 M using distilled water, a 100 mL volumetric flask, and a pipet or buret. Include specific amounts where appropriate.
The student takes the 0.10 M solution and determines the percent transmittance and the absorbance at various wavelengths. The two graphs below represent the data.
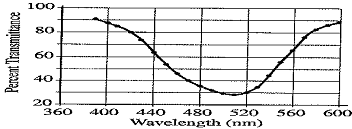
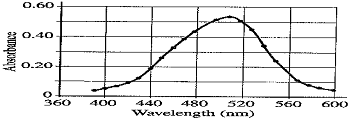
(b) Identify the optimum wavelength for the analysis.
The student measures the absorbance of the 0.020 M, 0.040 M, 0.060 M, 0.080 M and 0.10 M solutions. The data are plotted below.
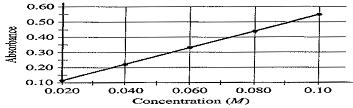
(c) The absorbance of the unknown solution is 0.275. What is the concentration of the solution?
(d) Beer’s Law is an expression that includes three factors that determine the amount of light that passes through a solution. Identify two of these factors.
(e) The student handles the sample container (e.g., test tube or cuvette) that holds the unknown solution and leaves fingerprints in the path of the light beam. How will this affect the calculated concentration of the unknown? Explain your answer.
(f) Why is this method of determining the concentration of CoCl2 solution appropriate, whereas using the same method for measuring the concentration of NaCl solution would not be appropriate?
Answer:
(a) M1V1 = M2V2; (0.10M)(V1) = (0.020M)(100. mL)
V1 = 20.0 mL
a 20.0 mL aliquot of 0.10 M solution is measured by buret or pipet. this aliquot is added to the 100-mL volumetric flask and filled, with mixing, to the line on the neck with distilled water
(b) approx. 510 nm
(c) approx. 0.05 M
(d) extinction coefficient
path length of light
concentration of absorbing species
(e) fingerprints scatter light and the detector gets less light, the reading of absorbance is higher, indicating a higher than expected concentration
(f) the Na+ ion does not absorb energy in the visible spectrum, whereas the Co2+ is a rose color
2003 D Required
6. For each of the following, use appropriate chemical principles to explain the observations. Include chemical equations as appropriate.
(a) In areas affected by acid rain, statues and structures made of limestone (calcium carbonate) often show signs of considerable deterioration.
(b) When table salt (NaCl) and sugar (C12H22O11) are dissolved in water, it is observed that
(i) both solution have higher boiling points than pure water, and
(ii) the boiling point of 0.10 M NaCl(aq) is higher than that of 0.10 M C12H22O11(aq).
(c) Methane gas does not behave as an ideal gas at low temperatures and high pressures.
(d) Water droplets form on the outside of a beaker containing an ice bath.
Answer:
(a) limestone reacts with acid to produce a soluble substance, a gas, and water which wash away
CaCO3(s) + H+(aq) ® Ca2+(aq) + CO2(g) + H2O(l)
(b) (i) a solution made from a non-volatile solute has a higher boiling point than the pure solvent because the solution has a lower vapor pressure than the water (Raoult’s Law) . the temperature of the solution has be higher to produce enough vapor pressure to equal the atmospheric pressure (i.e., boiling)
(ii) the amount of boiling point elevation depends on the number of non-volatile particles in solution. since the salt dissociates into 2 particles for every NaCl that dissolves, it will increase the boiling point more that an equal concentration of sugar (a molecular cpd) that does not dissociate or ionize.
(c) at low temperatures and high pressures, the methane molecules are slow and closer together. under these conditions, van der Waal forces become measurable and significant and creates a deviation from ideal behavior. at high pressure the volume of a real molecule is also significant.
(d) a water vapor molecules collide with the cool beaker, the molecules lose kinetic energy, slow down, attract others, and condense into a liquid
Answer EITHER Question 7 below OR Question 8. Only one of these two questions will be graded. If you start both questions, be sure to cross out the question you do not want graded. The Section II score weighting for the question you choose is 15 percent.
2003 D
7. Answer the following questions that relate to the chemistry of nitrogen.
(a) Two nitrogen atoms combine to form a nitrogen molecule, as represented by the following equation.
2 N(g) ® N2(g)
Using the table of average bond energies below, determine the enthalpy change, ∆H, for the reaction.
|
Bond |
Average Bond Energy (kJ mol–1) |
|
N–N |
160 |
|
N=N |
420 |
|
NºN |
950 |
(b) The reaction between nitrogen and hydrogen to form ammonia is represented below.
N2(g) + 3 H2(g) ® 2 NH3(g) ∆H˚ = –92.2 kJ
Predict the sign of the standard entropy change, ∆S˚, for the reaction. Justify your answer.
(c) The value of ∆G˚ for the reaction represented in part (b) is negative at low temperatures but positive at high temperatures. Explain.
(d) When N2(g) and H2(g) are placed in a sealed container at a low temperature, no measurable amount of NH3(g) is produced. Explain.
Answer:
(a) a triple bond is formed, an exothermic process
∆H = –950 kJ mol–1
(b) (–); the mixture of gases (high entropy) is converted into a pure gas (low entropy) and the 4 molecules of gas is reduced to 2, a smaller number of possible microstates is available
(c) ∆G˚ = ∆H˚ – T∆S˚; enthalpy favors spontaneity (∆H < 0), negative entropy change does not favor spontaneity. Entropy factor becomes more significant as temperature increases. At high temperatures the T∆S factor becomes larger in magnitude than ∆H and the reaction is no longer spontaneous (∆G > 0).
(d) at low temperatures, the kinetic energy of the molecules is low and very few molecules have enough activation energy
2003 D
|
Compound Name |
Compound Formula |
∆H˚vap (kJ mol-1) |
|
Propane |
CH3CH2CH3 |
19.0 |
|
Propanone |
CH3COCH3 |
32.0 |
|
1-propanol |
CH3CH2CH2OH |
47.3 |
8. Using the information in the table above, answer the following questions about organic compounds.
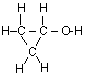
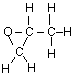
(a) For propanone,
(i) draw the complete structural formula (showing all atoms and bonds);
(ii) predict the approximate carbon-to-carbon-to-carbon bond angle.
(b) For each pair of compounds below, explain why they do not have the same value for their standard heat of vaporization, ∆H˚vap. (You must include specific information about both compounds in each pair.)
(i) Propane and propanone
(ii) Propanone and 1-propanol
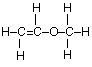
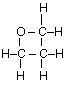
(c) Draw the complete structural formula for an isomer of the molecule you drew in, part (a) (i).
(d) Given the structural formula for propyne below,
H
| ¯
H—C—CºC—H
|
H
(i) indicate the hybridization of the carbon atom indicated by the arrow in the structure above;
(ii) indicate the total number of sigma (s) bands and the total number of pi (π) bonds in the molecule
Answer:
(a) (i) 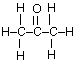
(ii) 120˚
(b) (i) propane, 26 electrons, molar mass = 44
propanone, 32 electrons, molar mass = 58
higher # electrons means larger van der Waal forces, larger molar mass means a slower molecule, the oxygen creates a polar molecule and dipol–dipole interactions
(ii) 1-propanol has an –OH which creates a site for hydrogen bonding with other –OH on adjacent molecules increasing intermolecular forces that must be overcome in order to vaporize the liquid.
(c) 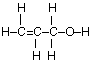
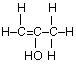
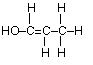
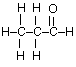
(d) (i) sp
(ii) 6 sigma, 2 pi
= = = = = = = = = = = = = = = = = = = =
Harvey Gendreau
Unlimited Potential
(508) 877-8723
(419) 735-4782 (fax)
http://www.apchemistry.com
apchemtchr@aol.com
hgendreau@rcn.com